

Some of these may be functional, but many proteins fold once more to form a quaternary structure. The secondary structure of proteins is as a result of hydrogen bonding and the resulting polypeptide can either occur as a beta-pleated sheet or an alpha-helix structure.įurther bonding and folding of polypeptides result in a tertiary structure being formed. However, proteins are not functional at this primary level of structure. The first layer of a protein is the amino acids which bond together to form a linear chain known as a polypeptide. The side with the amino group is called the N-terminal while the other side is called the C-terminal. The way they bond together ensures that there is always an amino group on one end and a carboxyl group on the opposite end of the polypeptide chain. These form the primary structure of the protein, the polypeptide chain. These amino acids then bond to each other via covalent bonds called peptide bonds. The amino acids are assembled at the ribosome by tRNA molecules during translation of the genetic code. Some examples of amino acids that have this structure include methionine, tryptophan, and glycine.
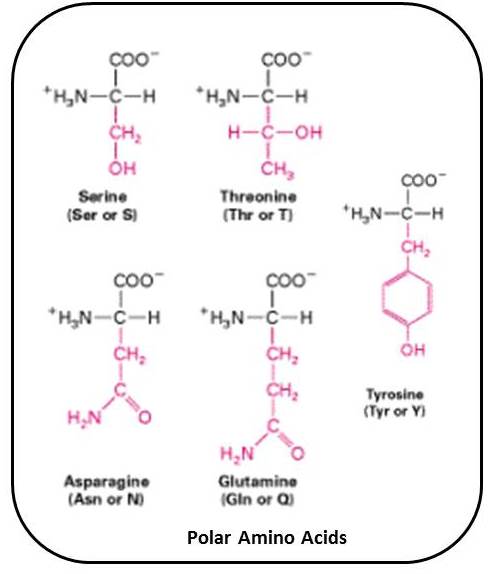
In the case where side chains are hydrophobic, they are found on the inside of the folded chain, away from water. The amino acids may have side chain components that are hydrophobic (repelling water) or hydrophilic (attracting water), which influence how they react with other molecules. Examples of these types of molecules include valine and leucine. Some amino acids are known as branched chain molecules because they have aliphatic side chains that are not linear. They can also be negatively charged acidic molecules such as aspartic acid or glutamic acid, or they can be positively charged basic molecules such as histidine and lysine. Glycine and serine are both amino acids that are uncharged polar molecules, while alanine and isoleucine are examples of nonpolar molecules. Amino acids vary based on what the side chain of the molecule is comprised of.ĭepending on the side chain, an amino acid can be a weak base or acid, and it can be nonpolar or polar. The central atom is an alpha carbon to which the amino and carboxyl groups are attached, as well as the R group. This molecule is made of a carboxyl group, an amino group and a side chain which is an organic R group. Amino acidsĪn amino acid is the simplest unit making up a protein. Many proteins also form hormones and allow cells to move. There are many functions of proteins including as receptors on cell membranes and as channels for substances to pass through into the cell.Īll enzymes are proteins that catalyze cellular reactions in the cell. Further bonding and folding results in a tertiary and quaternary protein structure being made.Ī protein is only functional if it is at the tertiary or quaternary level of structure. These are secondary structures that form. The polypeptides do bond further to form either a beta-pleated sheet or an alpha helix. The polypeptide chain that is made is the primary structure of the protein. They are brought together during the translation stage of protein synthesis.ĭuring this time the amino acids bond together covalently and peptide bonds form between them. In the case of a hydrophobic side group, this occurs on the inside of the chain away from water.Īmino acids can be charged and can be polar or nonpolar molecules. Diagram showing the general structure of an amino acid (Muellerb )Īmino acids can have hydrophilic or hydrophobic components. This R group is also called a side chain, and it varies from one acid to another. An amino acid consists of a central carbon atom to which are attached, a carboxyl group, an amino group, and an organic R group. The most basic unit of proteins is the amino acid molecule.


 0 kommentar(er)
0 kommentar(er)
